Title: A Partly Fermented Infant Formula with Postbiotics and Milk Fat Supports Adequate Growth, Is Safe and Well-Tolerated in Healthy Term Infants
| Authors: | Y. vandenPlas et al. |
| Published: | 2020 |
| Journal: | Nutrients |
A Partly Fermented Infant Formula with Postbiotics Including 3′-GL, Specific Oligosaccharides, 2′-FL, and Milk Fat Supports Adequate Growth, Is Safe and Well-Tolerated in Healthy Term Infants: A Double-Blind, Randomised, Controlled, Multi-Country Trial
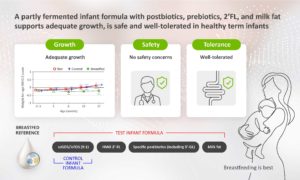
This recently published article in Nutrients (vandenPlas et al) concluded that a partly fermented infant formula with postbiotics, including 3’ -Galactosyllactose (3’ -GL), prebiotic mixture scGOS/lcFOS (9:1), 2’ -linked fucosyllactose (2’ -FL), and milk fat supports adequate growth, is safe and well-tolerated in healthy term infants.
This study was conducted in a double-blind, randomised, controlled, multi-country trial setting.
Read the full publication here.
In June 2018 the first healthy term infant was enrolled in the VOYAGE clinical study. The study took place in Belgium, Spain, Ukraine, Hungary and Poland. https://www.danoneresearch.com/news/first-infant-enrolled-for-the-voyage-study/